Difference between revisions of "IPLab:Lab 7:Malignant Melanoma"
Seung Park (talk | contribs) |
(→Images) |
||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
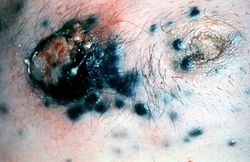
File:IPLab7Melanoma1.jpg|This is a gross photograph of skin with melanoma. Note the black pigment, multiple satellite nodules, and focal ulceration. Some of the satellite nodules affect the nipple. | File:IPLab7Melanoma1.jpg|This is a gross photograph of skin with melanoma. Note the black pigment, multiple satellite nodules, and focal ulceration. Some of the satellite nodules affect the nipple. | ||
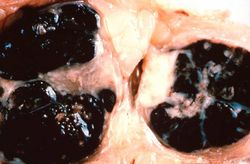
File:IPLab7Melanoma2.jpg|This is a gross photograph of lymph nodes almost entirely replaced by black pigment (melanin). | File:IPLab7Melanoma2.jpg|This is a gross photograph of lymph nodes almost entirely replaced by black pigment (melanin). | ||
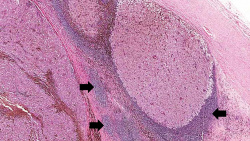
| − | File: | + | File:IPLab7Melanoma3b.jpg|This is a low-power photomicrograph of lymph node that is almost completely replaced/filled with tumor. This lymph node has a capsule (1) and some remaining lymphocytes (2) but the remainder of the node is replaced by tumor cells. |
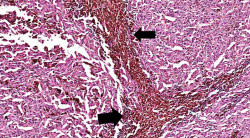
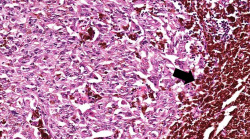
| − | File: | + | File:IPLab7Melanoma4b.jpg|This higher-power photomicrograph shows the remaining portion of lymph node (arrow). The rest of the lymph node is invaded by a neoplasm composed of cells with lighter eosinophilic cytoplasm and pigment. |
| − | File: | + | File:IPLab7Melanoma5b.jpg|This is a higher-magnification showing the abundant extracellular melanin (arrows) surrounding the tumor cells. This section of neoplasm shows the numerous cells with abundant cytoplasm and brown pigment within the cytoplasm of some of these cells. |
| − | File: | + | File:IPLab7Melanoma6b.jpg|This is a higher magnification showing the abundant extracellular melanin surrounding the tumor cells (brown pigment). |
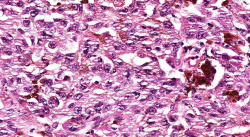
| − | File: | + | File:IPLab7Melanoma7b.jpg|This is a high-power photomicrograph of the main tumor mass with the cells growing as poorly formed nests and sheets of cells. There is little if any pigment in this section. |
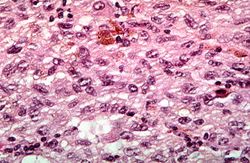
File:IPLab7Melanoma8.jpg|This is a high-power photomicrograph of the main tumor mass showing the cellular details. The individual melanoma cells contain large nuclei with irregular contours having chromatin clumped at the periphery of the nuclear membrane and prominent red (eosinophilic) nucleoli. | File:IPLab7Melanoma8.jpg|This is a high-power photomicrograph of the main tumor mass showing the cellular details. The individual melanoma cells contain large nuclei with irregular contours having chromatin clumped at the periphery of the nuclear membrane and prominent red (eosinophilic) nucleoli. | ||
</gallery> | </gallery> | ||
| + | |||
| + | == Virtual Microscopy == | ||
| + | <peir-vm>IPLab7Melanoma</peir-vm> | ||
== Study Question == | == Study Question == | ||
| Line 18: | Line 21: | ||
* <spoiler text="What are risk factors for developing melanoma?">Sunlight appears to play an important role in the development of skin malignant melanoma. Lightly pigmented individuals are at higher risk for the development of melanoma than darkly pigmented individuals. Sunlight does not seem to be the only predisposing factor, and the presence of a pre-existing nevus (e.g., a dysplastic nevus), hereditary factors, or even exposure to certain carcinogens may play a role in lesion development and evolution.</spoiler> | * <spoiler text="What are risk factors for developing melanoma?">Sunlight appears to play an important role in the development of skin malignant melanoma. Lightly pigmented individuals are at higher risk for the development of melanoma than darkly pigmented individuals. Sunlight does not seem to be the only predisposing factor, and the presence of a pre-existing nevus (e.g., a dysplastic nevus), hereditary factors, or even exposure to certain carcinogens may play a role in lesion development and evolution.</spoiler> | ||
* <spoiler text="What role may tumor specific antigens play in development of malignant melanoma?">Approximately 40% of human melanomas express a tumor specific antigen referred to as melanoma antigen-1 (MAGE-1). Molecular analysis has revealed that the gene encoding MAGE-1 is present in normal cells as well as in tumor cells, and there is no evidence that it is mutated in cancer cells. However, as has been seen in some animal tumors, the gene is silent in normal adult cells; whether or not it is expressed during development remains to be determined. CD8+ cytotoxic T cells specific for MAGE-1 can be obtained by culturing tumor cells with patients’ lymphocytes in vitro.</spoiler> | * <spoiler text="What role may tumor specific antigens play in development of malignant melanoma?">Approximately 40% of human melanomas express a tumor specific antigen referred to as melanoma antigen-1 (MAGE-1). Molecular analysis has revealed that the gene encoding MAGE-1 is present in normal cells as well as in tumor cells, and there is no evidence that it is mutated in cancer cells. However, as has been seen in some animal tumors, the gene is silent in normal adult cells; whether or not it is expressed during development remains to be determined. CD8+ cytotoxic T cells specific for MAGE-1 can be obtained by culturing tumor cells with patients’ lymphocytes in vitro.</spoiler> | ||
| + | |||
| + | == Additional Resources == | ||
| + | === Reference === | ||
| + | * [http://emedicine.medscape.com/article/1100753-overview eMedicine Medical Library: Cutaneous Melanoma] | ||
| + | * [http://emedicine.medscape.com/article/280245-overview eMedicine Medical Library: Malignant Melanoma] | ||
| + | * [http://emedicine.medscape.com/article/846566-overview eMedicine Medical Library: Skin Cancer -- Melanoma] | ||
| + | * [http://www.merckmanuals.com/professional/dermatologic_disorders/cancers_of_the_skin/melanoma.html Merck Manual: Melanoma] | ||
| + | |||
| + | === Journal Articles === | ||
| + | * Banerjee SS, Harris M. [http://www.ncbi.nlm.nih.gov/pubmed/10792480 Morphological and immunophenotypic variations in malignant melanoma]. ''Histopathology'' 2000 May;36(5):387-402. | ||
| + | * Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, Dummer R, North J, Pincus L, Ruben B, Rickaby W, D’Arrigo C, Robson A, and Bastian BC.[http://www.nejm.org/doi/pdf/10.1056/NEJMoa1502583 The Genetic Evolution of Melanoma from Precursor Lesions]. ''New England Journal of Medicine'' 2015 Nov;373:1926-1936. | ||
| + | |||
| + | === Images === | ||
| + | * [{{SERVER}}/library/index.php?/tags/2148-melanoma PEIR Digital Library: Melanoma Images] | ||
| + | * [http://library.med.utah.edu/WebPath/NEOHTML/NEOPLIDX.html WebPath: Neoplasia] | ||
{{IPLab 7}} | {{IPLab 7}} | ||
[[Category: IPLab:Lab 7]] | [[Category: IPLab:Lab 7]] | ||
Latest revision as of 02:14, 9 July 2020
Contents
Clinical Summary[edit]
This 68-year-old white male had a local excision of a pigmented lesion (melanoma) on the skin of his back. Three years later he became aware of a "lump" in his left axilla. Examination confirmed the presence of a 2.3-cm nodular lesion. Subsequently, the patient underwent a surgical procedure for removal of axillary lymph nodes.
Images[edit]
Virtual Microscopy[edit]
Study Question[edit]
Additional Resources[edit]
Reference[edit]
- eMedicine Medical Library: Cutaneous Melanoma
- eMedicine Medical Library: Malignant Melanoma
- eMedicine Medical Library: Skin Cancer -- Melanoma
- Merck Manual: Melanoma
Journal Articles[edit]
- Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology 2000 May;36(5):387-402.
- Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, Dummer R, North J, Pincus L, Ruben B, Rickaby W, D’Arrigo C, Robson A, and Bastian BC.The Genetic Evolution of Melanoma from Precursor Lesions. New England Journal of Medicine 2015 Nov;373:1926-1936.
Images[edit]
Nodular hyperplasia of the prostate--characterized by large discrete prostatic nodules--is a common disorder in men over 50 years of age. The nodules cause the prostate to be enlarged and to have an increased weight. The human prostate is surrounded by a restrictive capsule. These nodules cause increased pressure within the capsule which leads to constriction of the urethra as it passes through the prostate. Urethral constriction leads to retention of urine.