Clinical Summary[edit]
This 56-year-old white male came to the emergency room because of weakness, lack of appetite, shortness of breath, abdominal distention, and an altered mental status. He was a known to have alcohol use disorder and he drank approximately one pint of whiskey per day. Physical examination revealed a protuberant abdomen, bilateral gynecomastia, and spider angiomata on his chest. Liver enzymes were elevated, albumin was low and he was anemic.
The patient was given thiamine, folate, multivitamins, and vitamin K and an intravenous line was placed to infuse 5% dextrose. An esophagogastroduodenoscopy (EGD) demonstrated large esophageal varices. Two days after admission the patient developed a massive hematemesis due to rupture of an esophageal varices and despite successful sclerotherapy and supportive transfusions, the patient died the next day.
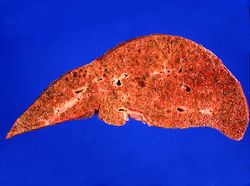
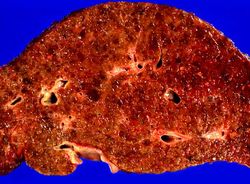
This is a gross photograph of the liver from this patient. Note the nodular pattern and the areas of greenish discoloration as well as pale tan areas.
This is a closer view of the liver from this patient. Again note the nodular pattern and the areas of greenish discoloration. These green nodules are actually the viable hepatocytes that are stained green because of bile stasis. The pale areas are the areas of fibrosis.
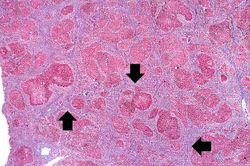
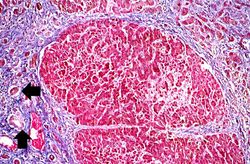
This is a low-power photomicrograph of this liver stained with a trichrome stain to highlight the fibrous tissue (arrows). Also note the nodular pattern.
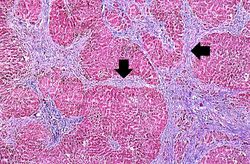
In this is medium-power photomicrograph of trichrome stained liver the bands of fibrous tissue are seen to form "bridges" between triad areas (arrows); this is called "bridging fibrosis." Also note the fibrous tissue (arrows) and how the hepatocytes are separated into nodules by this fibrous tissue.
In this high-power photomicrograph of trichrome-stained liver, the bands of fibrous tissue surround the hepatocyte nodules. There is some degeneration and dropout of hepatocytes in this nodule. Also note the increased numbers of bile ducts in the triad area (arrows). Bile duct proliferation is a common feature in many hepatitides.
This photograph from another autopsy case shows another example of cirrhosis. Note the nodules, the fibrosis, the green coloration and the small size of this cirrhotic liver.
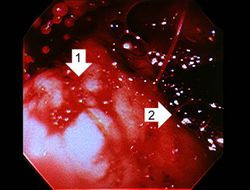
This photograph was taken during the EGD while the patient was alive. Note the red hyperemic areas (1) and the area of hemorrhage (2).
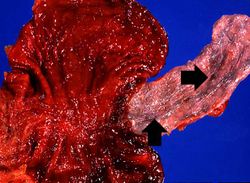
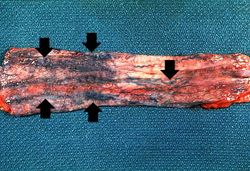
This photograph taken at autopsy shows the distal portion of the esophagus and the stomach. The esophageal varices are visible just under the mucosa of the esophagus (arrows). Some of the blood from the ruptured varix can still be seen in the stomach.
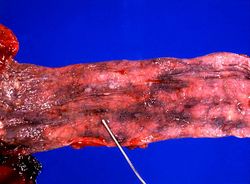
In this closer view of the distal esophagus the ruptured varix is indicated by the probe. Other varices and areas of submucosal hemorrhage can also be appreciated.
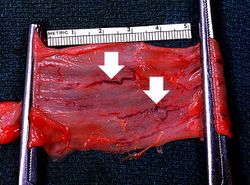
This is a photograph taken from another patient at autopsy to demonstrate numerous esophageal varices in the distal esophagus (arrows). None of these varices have ruptured.
This photograph taken from still another patient at autopsy demonstrates the esophageal varices in the distal esophagus (arrows). The esophagus was clamped before removing the esophagus from the body in order to trap the blood in these distended varices. It is obvious how easily these thin-walled superficial varices could rupture and bleed.
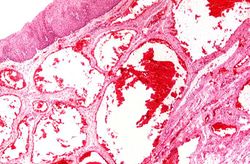
This photomicrograph shows the dilated vessel just under the epithelium of the esophagus.
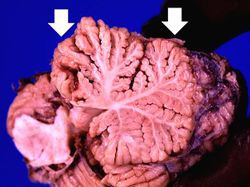
This photograph of the cerebellum from this patient demonstrates the marked thinning of the anterior portion of the cerebellum (arrows).
This is another photograph of the cerebellum from this patient demonstrating the marked thinning of the anterior portion of the cerebellum (arrows). This pattern of cerebellar damage is consistent with Wernicke's encephalopathy.
Study Questions[edit]
Initial changes include hepatocellular steatosis. This is caused by altered metabolism with high levels of NADH from lactate dehydrogenase resulting in increased lipid biosynthesis. Mobilization of lipids from peripheral fat stores and decreased lipid acceptor protein synthesis leads to insufficient lipoprotein production.
Alcohol induces free radical production as it is broken down by the microsomal ethanol oxidizing system. Alcohol also impairs microtubular and mitochondrial function and membrane fluidity.
Acetaldehyde, the major ethanol metabolite, can cause lipid peroxidation and acetaldehyde-protein complexes that further inhibit the microtubular system.
Finally, alcohol also induces an immunologic reaction in the liver. This immune-mediated liver damage is thought to result from the expression of neoantigens on hepatocytes possibly due to alcohol-induced alterations in membranes or acetaldehyde binding to proteins leading to neoantigen formation.
Since most of the clotting factors are produced by the liver, chronic liver damage with loss of liver parenchyma will lead to a reduction in clotting factors. In addition, this patient had bleeding esophageal varices and ascites which could both use up or sequester clotting factors, respectively.
Hepatic cirrhosis with extensive parenchymal damage and fibrosis results in an increased resistance to portal blood flow. The increased portal pressure leads to increased pressure in the coronary veins of the stomach. This results in increased pressure in the esophageal plexus in the terminal portion of the esophagus as the blood travels through this plexus to empty into the azygous vein. The increased pressure and increased flow of blood through this plexus of thin-walled veins leads to dilation and formation of varices. These varices can then rupture and lead to life-threatening hemorrhage as was seen in this case.
Increased portal pressure also leads to increased pressure in the inferior hemorrhoidal veins and can lead to the formation of anorectal varices.
Wernicke's encephalopathy is caused by thiamine (Vitamin B1) deficiency. Chronic alcoholics often have poor diets and alcohol inhibits intestinal absorption of thiamine. Thus, some chronic alcoholics can develop Wernicke's encephalopathy which consists of foci of symmetric discoloration, softening, and punctate hemorrhages in the paraventricular regions of the thalamus and hypothalamus, in the mamillary bodies, around the aqueduct in the midbrain, in the floor of the fourth ventricle and in the anterior cerebellum. There is demyelinization and loss of neuropil. Even after treatment with thiamine, there is significant memory deficit.
Additional Resources[edit]
Reference[edit]
Journal Articles[edit]
Related IPLab Cases[edit]