Difference between revisions of "IPLab:Lab 1:Myocardial Infarction"
Seung Park (talk | contribs) |
Seung Park (talk | contribs) |
||
| Line 28: | Line 28: | ||
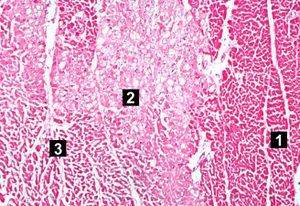
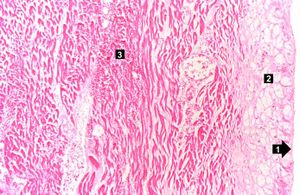
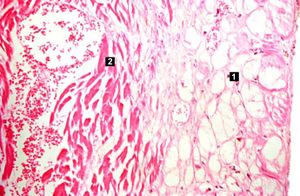
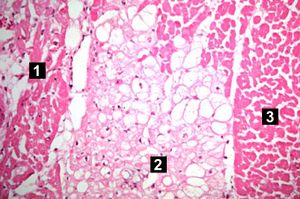
| This high-power photomicrograph contains normal myocytes (1), vacuolated myocytes (2), and infarcted myocytes (3). | | This high-power photomicrograph contains normal myocytes (1), vacuolated myocytes (2), and infarcted myocytes (3). | ||
|} | |} | ||
| + | |||
| + | == Study Questions == | ||
| + | * What type of necrosis is present in this myocardial tissue? | ||
| + | |||
| + | * What are the morphologic characteristics of coagulative necrosis? | ||
| + | |||
| + | * What causes the vacuolar change seen in the tissue adjacent to this infarct and is this change reversible or irreversible injury? | ||
{{Template:IPLab 1}} | {{Template:IPLab 1}} | ||
[[Category:IPLab]] | [[Category:IPLab]] | ||
Revision as of 14:08, 15 August 2013
Clinical Summary[edit]
This was a 57-year-old male whose hospital course following abdominal surgery was characterized by progressive deterioration and hypotension. Four days post-operatively, the patient sustained an anterior myocardial infarction and died the next day.
Autopsy Findings[edit]
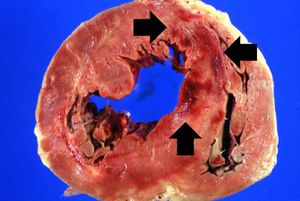
The patient's heart weighed 410 grams. Examination of the coronary arteries revealed marked atherosclerotic narrowing of all three vessels with focal occlusion by a thrombus of the left anterior descending artery. Fresh necrosis of the anterior wall of the left ventricle and anterior portion of the septum was present, extending from the endocardium to the inner half of the ventricular wall.
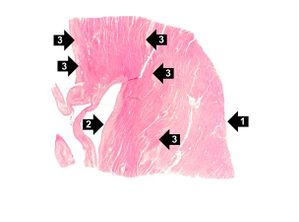
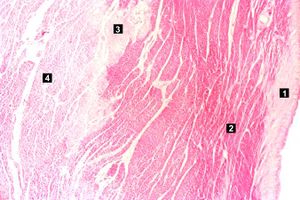
Images[edit]
Study Questions[edit]
- What type of necrosis is present in this myocardial tissue?
- What are the morphologic characteristics of coagulative necrosis?
- What causes the vacuolar change seen in the tissue adjacent to this infarct and is this change reversible or irreversible injury?
| |||||
Myocardial infarction is necrosis of myocardial tissue which occurs as a result of a deprivation of blood supply, and thus oxygen, to the heart tissue. Blockage of blood supply to the myocardium is caused by occlusion of a coronary artery.
A normal heart weighs 300 grams (range: 270 to 360 grams).
Atherosclerosis is the deposition of lipid into the intima of arteries, resulting in narrowing of the vessel lumen.
An occlusion is a blockage.
A thrombus is a solid mass resulting from the aggregation of blood constituents within the vascular system.